Exploring the Ultraviolet Catastrophe in Blackbody Radiation
Written on
Chapter 1: Understanding Blackbody Radiation
Welcome to our exploration of blackbody radiation, a concept significantly impacted by the ultraviolet catastrophe. This article is primarily authored by Benedict Goh, a dedicated physics student I've collaborated with. Let’s dive into this fascinating topic!
The Concept of a Blackbody
Imagine a perfectly sealed box lined with reflective surfaces, akin to mirrors. Now, envision creating a small opening in one side of this box to allow energy to enter. Over time, the system reaches a state of 'thermal equilibrium,' where the energy is uniformly distributed within the box. This structure, absorbing light across the entire electromagnetic spectrum and emitting it at various frequencies, is termed a blackbody.
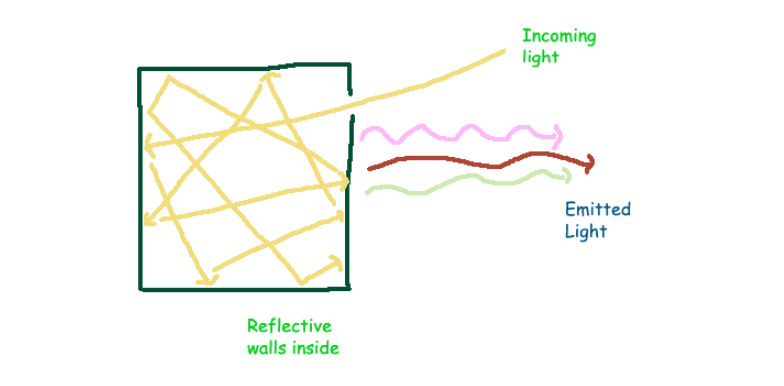
Within this blackbody, electromagnetic waves bounce off the walls. According to classical physics, these waves are described by Maxwell's equations, which impose certain boundary conditions at the walls, thereby limiting their possible frequencies. To maintain standing waves, the frequencies must accommodate an integer number of oscillations within the box.
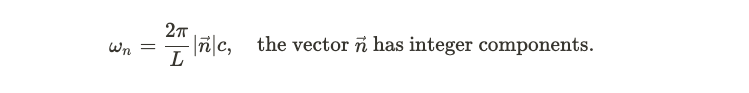
Intensity of Light Emission
In classical thermodynamics, the equipartition theorem plays a crucial role. In this framework, light is seen as a wave with varying frequencies, referred to as modes. The equipartition principle indicates that a blackbody emits energy uniformly across all modes. There is no distinction between high-frequency and low-frequency emissions, leading to a uniform distribution of light modes.
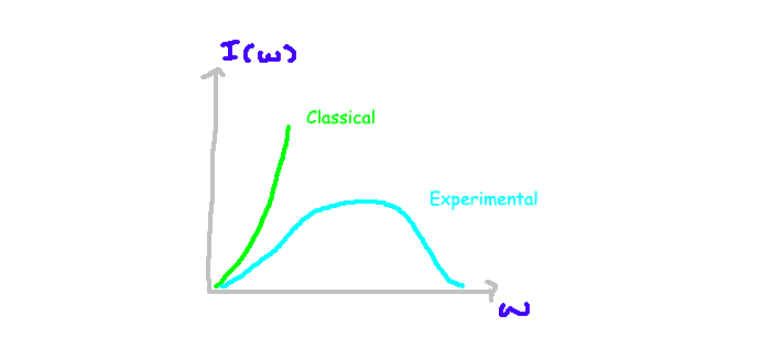
Mathematically, the emitted power per unit area and per frequency can be expressed, raising questions about how intensity varies with increasing energy. However, the classical model predicts a quadratic increase in intensity with frequency, resulting in an unrealistic explosion of intensity at high frequencies—something not observed in experiments.

The Discrepancy Between Theory and Reality
A blackbody is theoretically a perfect absorber and emitter of radiation across all frequencies. Yet, when experiments are conducted to measure the emitted intensity and frequency, the outcomes deviate from classical predictions. Researchers can prepare a blackbody and utilize detectors to measure its emitted radiation, plotting intensity against frequency to reveal a significant discrepancy from the classical model, particularly at higher frequencies.
Max Planck's Revolutionary Theory
In October 1900, Max Planck proposed a groundbreaking solution to the blackbody radiation dilemma. He suggested that energy in each electromagnetic mode is quantized, meaning it can only be emitted in discrete units, which he formulated using the Planck constant. This revolutionary postulate reshaped our understanding of light and energy.

By assuming that electromagnetic waves consist of quantized energy units, Planck likened light to currency—just as one cannot divide a coin into smaller denominations, energy cannot be infinitely subdivided.
Resolving the Ultraviolet Catastrophe
If Planck's theory holds true, we can reevaluate the intensity of light as a function of frequency. By calculating the thermal distribution and deriving an expression for intensity, we find that Planck's equation accurately fits experimental data, thus resolving the previously encountered ultraviolet catastrophe.
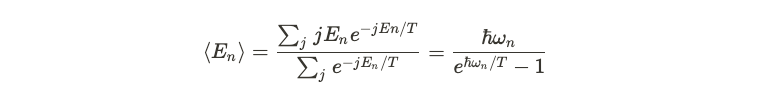
With these developments, Max Planck marked a pivotal moment in physics, being the first to successfully explain the blackbody radiation spectrum.
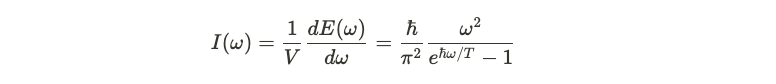
References
[2] Quantum Field Theory and the Standard Model, 1st Edition, Matthew D. Schwartz, HB ISBN: 9781107034730
Chapter 2: The Ultraviolet Catastrophe Explained
To further grasp the nuances of the ultraviolet catastrophe, let's watch the following video that illustrates its historical context and significance.
The first video, titled "The ULTRAVIOLET CATASTROPHE," provides an engaging overview of the phenomenon and its implications in physics.
Next, we will delve deeper into the specifics of the ultraviolet catastrophe and its resolution through Planck's theories.
The second video, "What is the Ultraviolet Catastrophe?" offers insights into the challenge and how it shaped modern physics.